This week in Part 6 of the Yarn Bleeding Experiment, we’re testing out adding vinegar to the bath.
If you haven’t already, head back and read Parts 1-5 first, so you know what’s going on.
Jump Links
Part 1: Introduction
Part 2: The Experiment
Part 3: Round One – Temperature
Part 4: Round Two – Wool Wash (Soak)
Part 5: Round Two – Wool Wash (Eucalan)
Part 6: Round Two – White Vinegar
Part 7: Round Two – Washing Up Liquid
Part 8: Round Three – Colour Catcher
Part 9: Results & Conclusion
Okay, all caught up? Great.
If you remember way back in Part 1, I talked about how dyeing yarn using acid dyes works. For a quick recap, acid dyes work when an acid is added to the dye bath. Professional dyers tend to use citric acid, but hobby dyers often get started with white vinegar.
This knowledge has given rise to the idea that adding vinegar to your blocking bath will stop the yarn from bleeding.
Looking at the science of it, there could be some truth there; after all, it is the vinegar (or citric acid) that lowers the pH of the dye bath, which is what allows dye to adhere to the yarn. Surely if we create the conditions required for dye to adhere, that might also prevent the opposite from happening?
Let’s test it!
So once again, I’m following the steps laid out in Part 2. After getting the big jug ready at 50C / 122F, I’ve added 5ml white vinegar – just the normal kind you buy at the supermarket. As usual, the temp is high to encourage bleeding, so we can be sure that any reduction in bleeding is attributed to the vinegar.
At this point I measured the pH of the water, and its hardness (TDS). As expected, the pH was low this time – 6.18. The surprise was the water hardness, which gave the highest measurement yet at 420 mg/L. Anything over 300 is considered “very hard”, but I was still a little surprised to see such a jump; the highest so far was 344 mg/L.
So far there has been a gentle but consistent correlation between water hardness and bleeding. The higher the TDS, the more bleeding seems to occur.
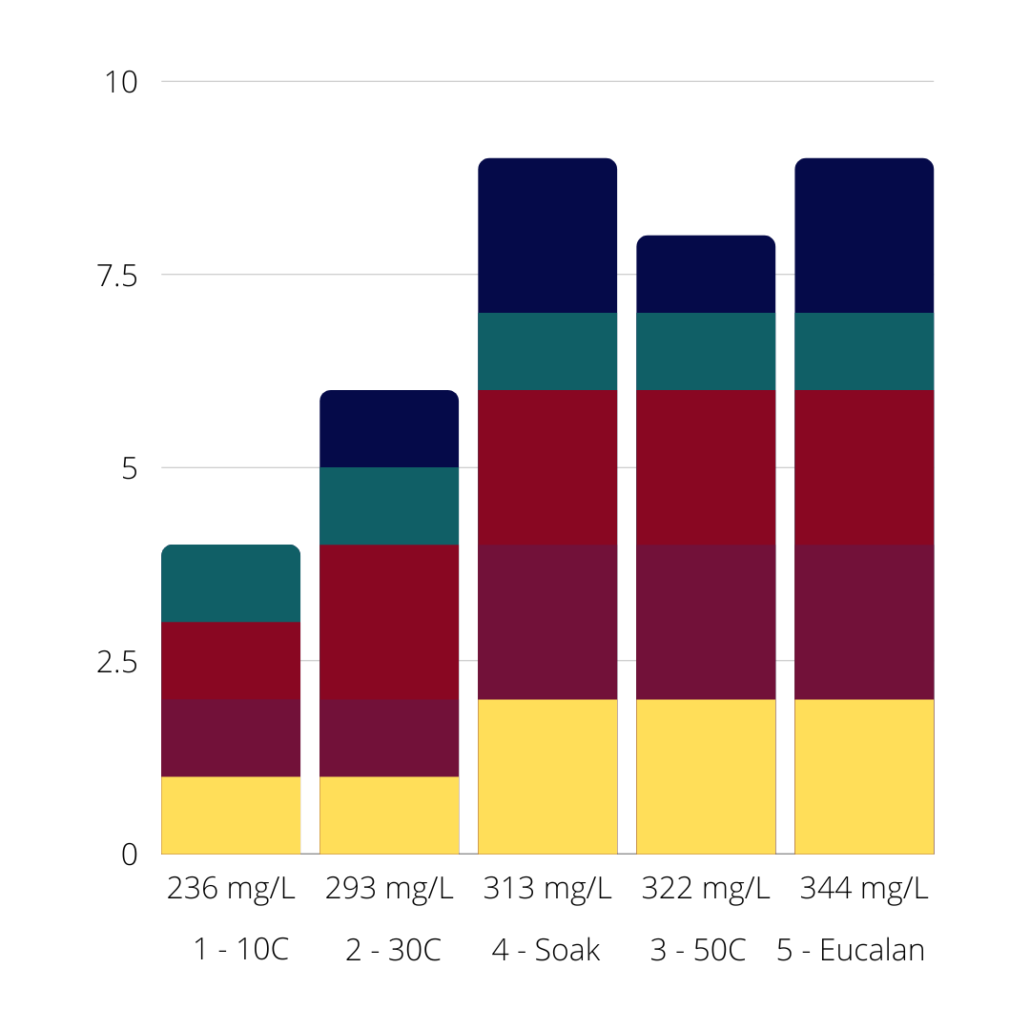
This chart shows the TDS values for each experiment so far along the X axis and the stacked total bleed values along the Y. The bleed values are colour coded so you can see how each individual colour bled.
As you can see, the general trend is the higher the TDS value, the more bleeding occurs.
So with this new, much higher TDS value, are we going to see a lot of bleeding here?
Time to split up the water and measure out 300ml for each blocking bath.
Half an hour later…
I’ve shown both the 50C and the 30C from Round One alongside the vinegar results, because the 30C was so similar, I wanted you to see them side by side. They are so similar that I’ve given them the exact same ratings as the 30C baths.
This means we have a couple of conclusions we can draw so far! Firstly, the hypothesis that adding vinegar to the bath will reduce bleeding does seem to have some truth to it; when comparing the vinegar bath on the right to the 50C bath on the left, there’s a little less bleeding across almost all the colours. However, my observation that higher TDS seems to lead to more bleeding has been blown out of the water with this result:
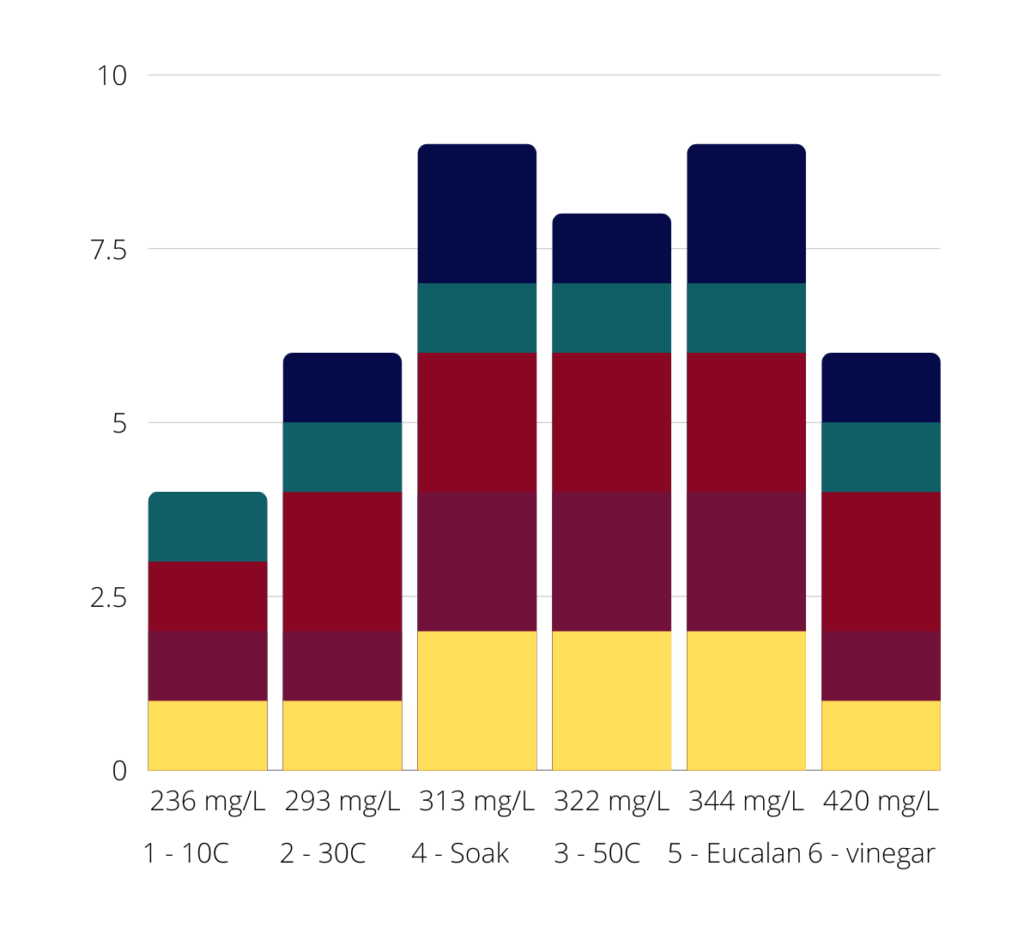
Well, that was a nice theory while it lasted!
That’s it for this instalment; next time I’ll be adding some washing up liquid (dish soap for my friends across the pond) to see what that does. Until then!












3 comments
Comments are closed.